Charting brain growth and aging at high spatial precision
- SaigeRutherford[email protected]112
- CharlotteFraza11
- RichardDinga11
- SeyedMostafaKia113
- ThomasWolfers44
- MariamZabihi11
- PierreBerthet44
- AmandaWorker5
- SerenaVerdi55
- DerekAndrews6
- LauraKMHan77
- JohannaMMBayer88
- PaolaDazzan55
- PhillipMcGuire5
- RoelTMocking7
- AartSchene11
- ChandraSripada2
- IvyFTso2
- ElizabethRDuval2
- Soo-EunChang2
- BrendaWJHPenninx77
- MaryMHeitzeg2
- SAlexandraBurt9
- LukeWHyde2
- DavidAmaral6
- ChristineWuNordahl6
- OleAAndreasssen44
- LarsTWestlye444
- RolandZahn5
- HenricusGRuhe11
- ChristianBeckmann1110
- AndreFMarquand11
- Short Report
- Neuroscience
- normative model
- lifespan
- growth chart
- brain chart
- big data
- individual prediction
- Human
- publisher-id72904
- doi10.7554/eLife.72904
- elocation-ide72904
Abstract
Defining reference models for population variation, and the ability to study individual deviations is essential for understanding inter-individual variability and its relation to the onset and progression of medical conditions. In this work, we assembled a reference cohort of neuroimaging data from 82 sites (N=58,836; ages 2–100) and used normative modeling to characterize lifespan trajectories of cortical thickness and subcortical volume. Models are validated against a manually quality checked subset (N=24,354) and we provide an interface for transferring to new data sources. We showcase the clinical value by applying the models to a transdiagnostic psychiatric sample (N=1985), showing they can be used to quantify variability underlying multiple disorders whilst also refining case-control inferences. These models will be augmented with additional samples and imaging modalities as they become available. This provides a common reference platform to bind results from different studies and ultimately paves the way for personalized clinical decision-making.
Introduction
Since their introduction more than a century ago, normative growth charts have become fundamental tools in pediatric medicine and also in many other areas of anthropometry 5Cole2012. They provide the ability to quantify individual variation against centiles of variation in a reference population, which shifts focus away from group-level (e.g., case-control) inferences to the level of the individual. This idea has been adopted and generalized in clinical neuroimaging, and normative modeling is now established as an effective technique for providing inferences at the level of the individual in neuroimaging studies 27Marquand et al.201628Marquand et al.2019.
Although normative modeling can be used to estimate many different kinds of mappings—for example between behavioral scores and neurobiological readouts—normative models of brain development and aging are appealing considering that many brain disorders are grounded in atypical trajectories of brain development 19Insel2014 and the association between cognitive decline and brain tissue in aging and neurodegenerative diseases 20Jack et al.201022Karas et al.2004. Indeed, normative modeling has been applied in many different clinical contexts, including charting the development of infants born pre-term 8Dimitrova et al.2020 and dissecting the biological heterogeneity across cohorts of individuals with different brain disorders, including schizophrenia, bipolar disorder, autism, and attention-deficit/hyperactivity disorder 3Bethlehem et al.202046Wolfers et al.202147Zabihi et al.2019.
A hurdle to the widespread application of normative modeling is a lack of well-defined reference models to quantify variability across the lifespan and to compare results from different studies. Such models should: (1) accurately model population variation across large samples; (2) be derived from widely accessible measures; (3) provide the ability to be updated as additional data come online, (4) be supported by easy-to-use software tools, and (5) should quantify brain development and aging at a high spatial resolution, so that different patterns of atypicality can be used to stratify cohorts and predict clinical outcomes with maximum spatial precision. Prior work on building normative modeling reference cohorts 4Bethlehem et al.2021 has achieved some of these aims (1–4), but has modeled only global features (i.e., total brain volume), which is useful for quantifying brain growth but has limited utility for the purpose of stratifying clinical cohorts (aim 5). The purpose of this paper is to introduce a set of reference models that satisfy all these criteria.
To this end, we assemble a large neuroimaging data set (Table 1) from 58,836 individuals across 82 scan sites covering the human lifespan (aged 2–100, Figure 1A) and fit normative models for cortical thickness and subcortical volumes derived from Freesurfer (version 6.0). We show the clinical utility of these models in a large transdiagnostic psychiatric sample (N=1985, Figure 2). To maximize the utility of this contribution, we distribute model coefficients freely along with a set of software tools to enable researchers to derive subject-level predictions for new data sets against a set of common reference models.
| N (subjects) | N (sites) | Sex (%F/%M) | Age (Mean, S.D) | ||
|---|---|---|---|---|---|
| Full | All | 58,836 | 82 | ||
| Training set | 29,418 | 82 | 51.1/48.9 | 46.9, 24.4 | |
| Test set | 29,418 | 82 | 50.9/49.1 | 46.9, 24.4 | |
| mQC | All | 24,354 | 59 | ||
| Training set | 12,177 | 59 | 50.2/49.8 | 30.2, 24.1 | |
| Test set | 12,177 | 59 | 50.4/49.4 | 30.1, 24.2 | |
| Clinical | Test set | 1985 | 24 | 38.9/61.1 | 30.5, 14.1 |
| Transfer | Test set | 546 | 6 | 44.5/55.5 | 24.8, 13.7 |
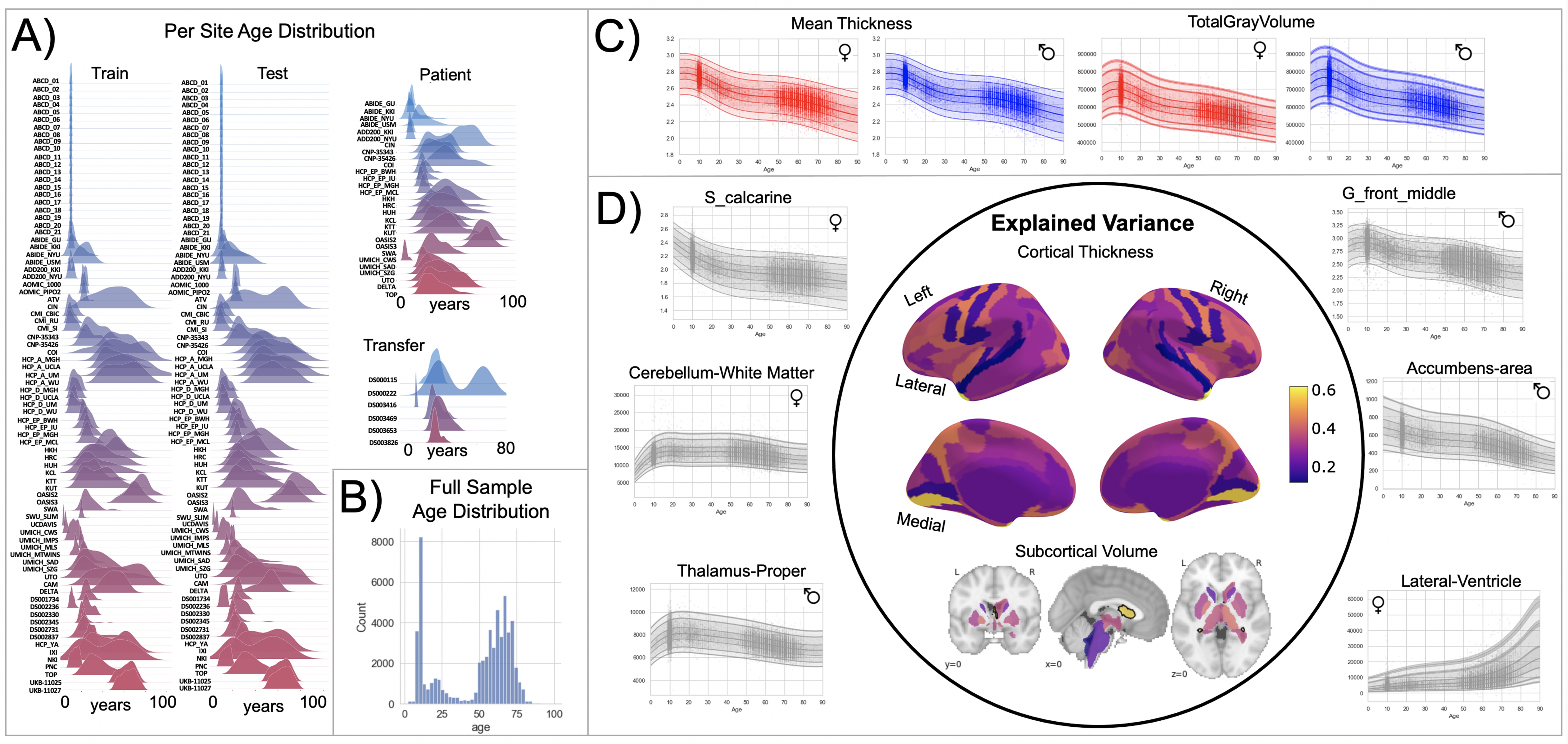
Normative model overview.
(A) Age density distribution (x-axis) of each site (y-axis) in the full model train and test, clinical, and transfer validation set. (B) Age count distribution of the full sample (N=58,836). (C, D) Examples of lifespan trajectories of brain regions. Age is shown on x-axis and predicted thickness (or volume) values are on the y-axis. Centiles of variation are plotted for each region. In (C), we show that sex differences between females (red) and males (blue) are most pronounced when modeling large-scale features such as mean cortical thickness across the entire cortex or total gray matter volume. These sex differences manifest as a shift in the mean in that the shape of these trajectories is the same for both sexes, as determined by sensitivity analyses where separate normative models were estimated for each sex. The explained variance (in the full test set) of the whole cortex and subcortex is highlighted inside the circle of (D). All plots within the circle share the same color scale. Visualizations for all ROI trajectories modeled are shared on GitHub for users that wish to explore regions not shown in this figure.
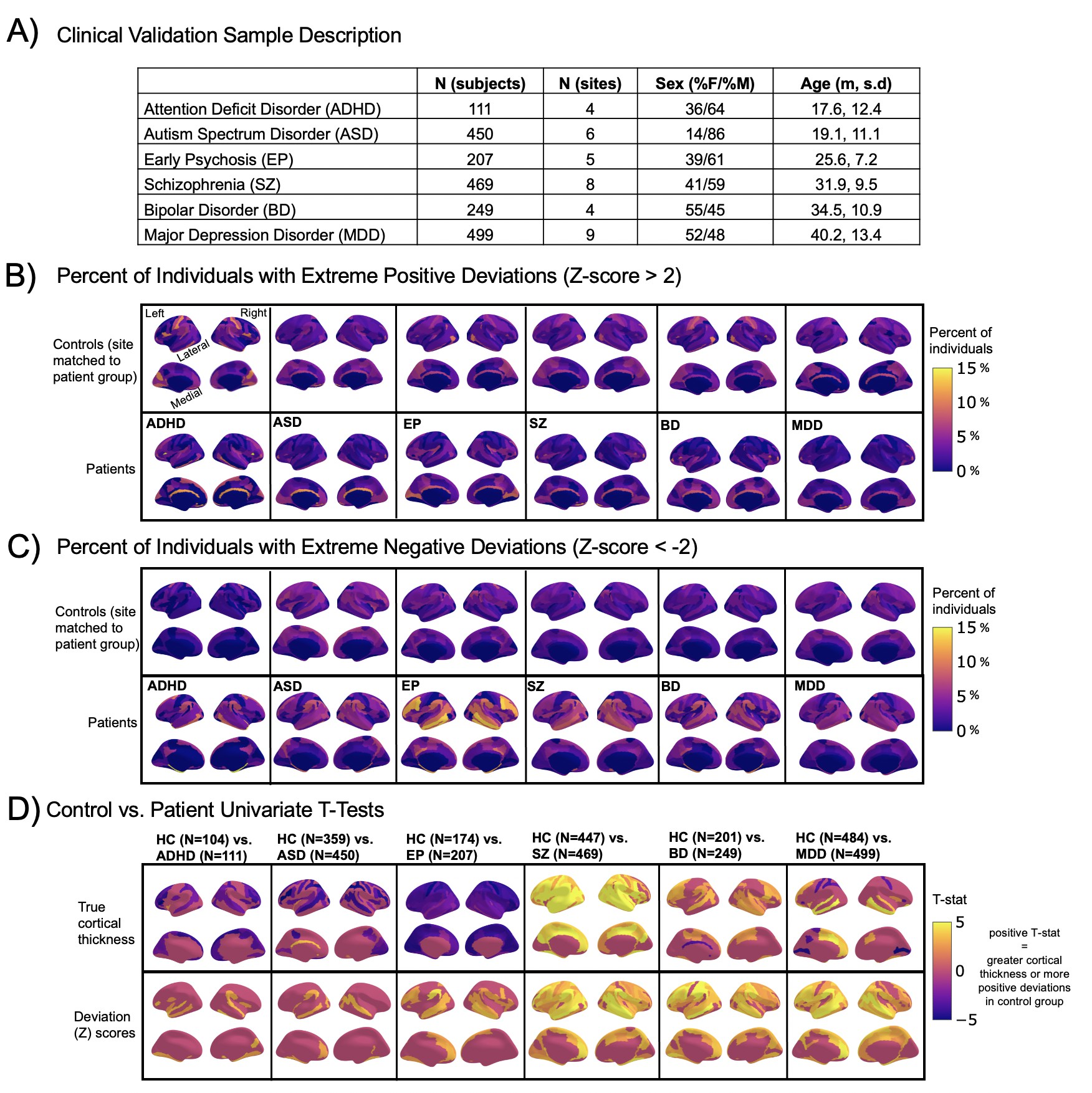
Normative modeling in clinical cohorts.
Reference brain charts were transferred to several clinical samples (described in (A)). Patterns of extreme deviations were summarized for each clinical group and compared to matched control groups (from the same sites). (B) Shows extreme positive deviations (thicker/larger than expected) and (C) shows the extreme negative deviation (thinner/smaller than expected) patterns. (D) Shows the significant (FDR corrected p<0.05) results of classical case-control methods (mass-univariate t-tests) on the true cortical thickness data (top row) and on the deviations scores (bottom row). There is unique information added by each approach which becomes evident when noticing the maps in (B–D) are not identical. ADHD, attention-deficit hyperactive disorder; ASD, autism spectrum disorder; BD, bipolar disorder; EP, early psychosis; FDR, false discovery rate; MDD, major depressive disorder; SZ, schizophrenia.
Results
We split the available data into training and test sets, stratifying by site (Table 1, Supplementary files 1 and 2). After careful quality checking procedures, we fit normative models using a set of covariates (age, sex, and fixed effects for site) to predict cortical thickness and subcortical volume for each parcel in a high-resolution atlas 7Destrieux et al.2010. We employed a warped Bayesian linear regression model to accurately model non-linear and non-Gaussian effects 14Fraza et al.2021, whilst accounting for scanner effects 2Bayer et al.202123Kia et al.2021. These models are summarized in Figure 1 and Figure 3, Figure 3—figure supplements 1–3, and with an online interactive visualization tool for exploring the evaluation metrics across different test sets (overview of this tool shown in Video 1). The raw data used in these visualizations are available on GitHub 36Rutherford2022.
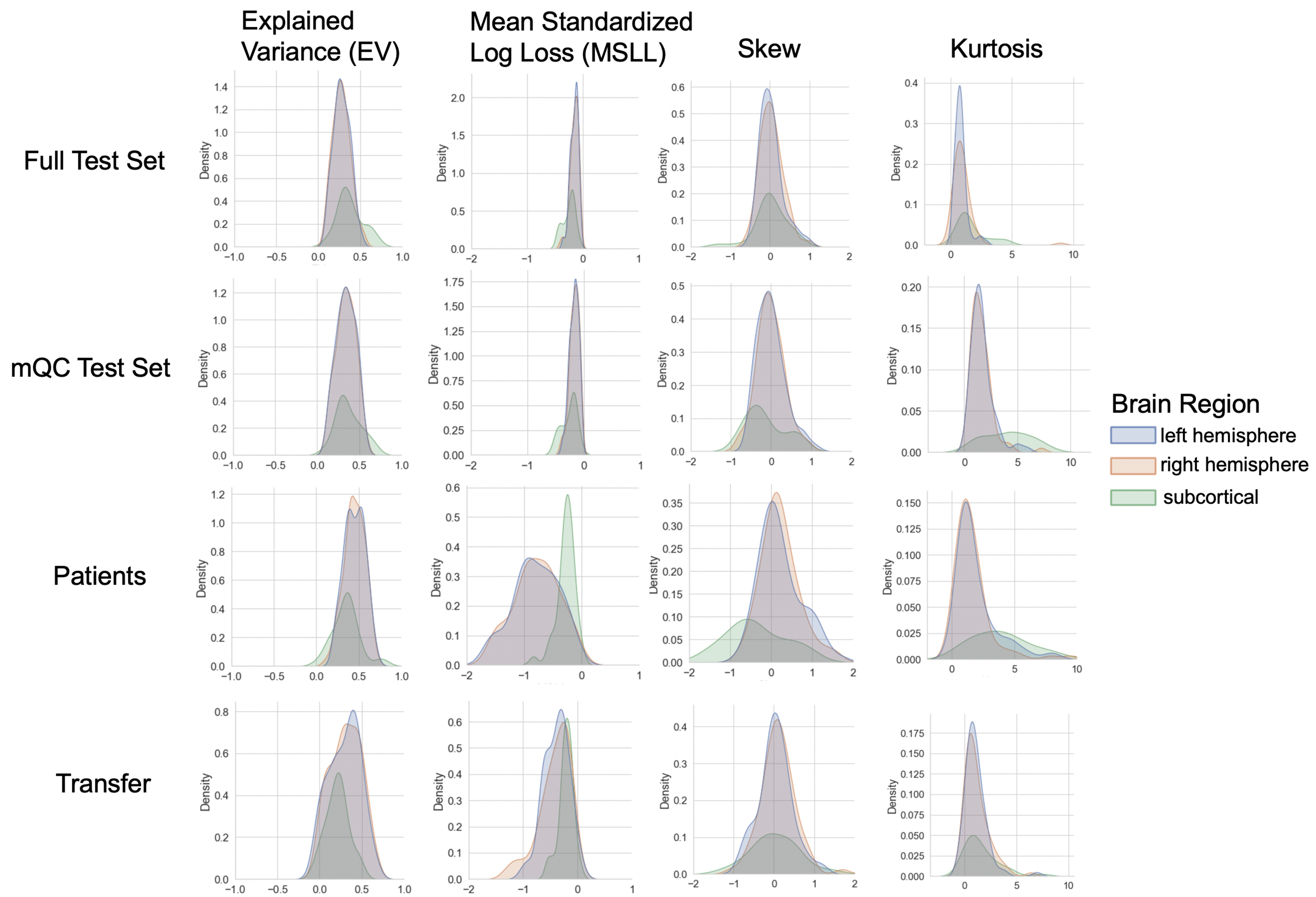
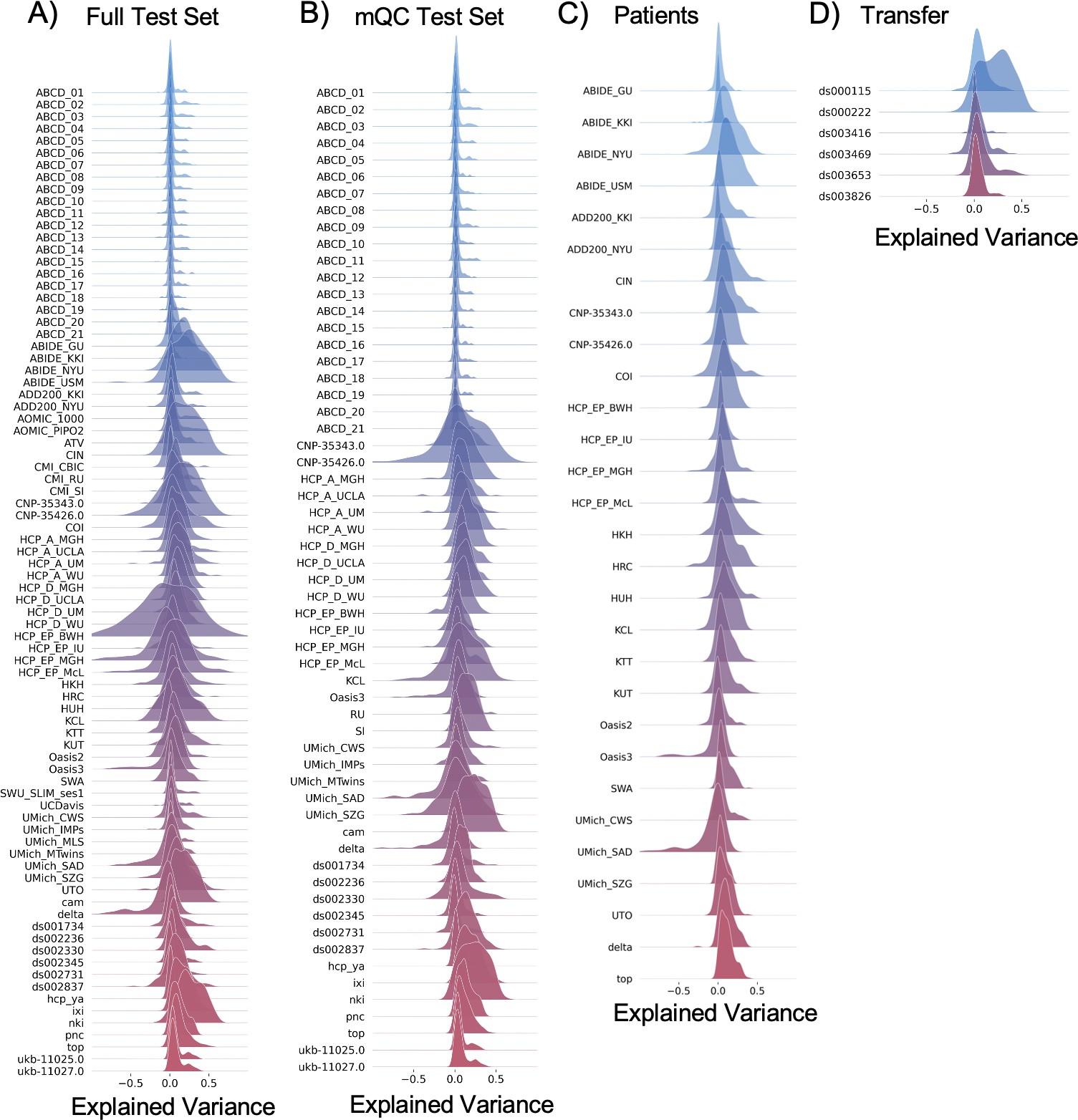
Evaluation metrics across all test sets.
The distribution of evaluation metrics in four different test sets (full, mQC, patients, and transfer, see Materials and methods) separated into left and right hemispheres and subcortical regions, with the skew and excess kurtosis being measures that depict the accuracy of the estimated shape of the model, ideally both would be around zero. Note that kurtosis is highly sensitive to outlying samples. Overall, these models show that the models fit well in term of central tendency and variance (explained variance and MSLL) and model the shape of the distribution well in most regions (skew and kurtosis). Code and sample data for transferring these models to new sites not included in training is shared.
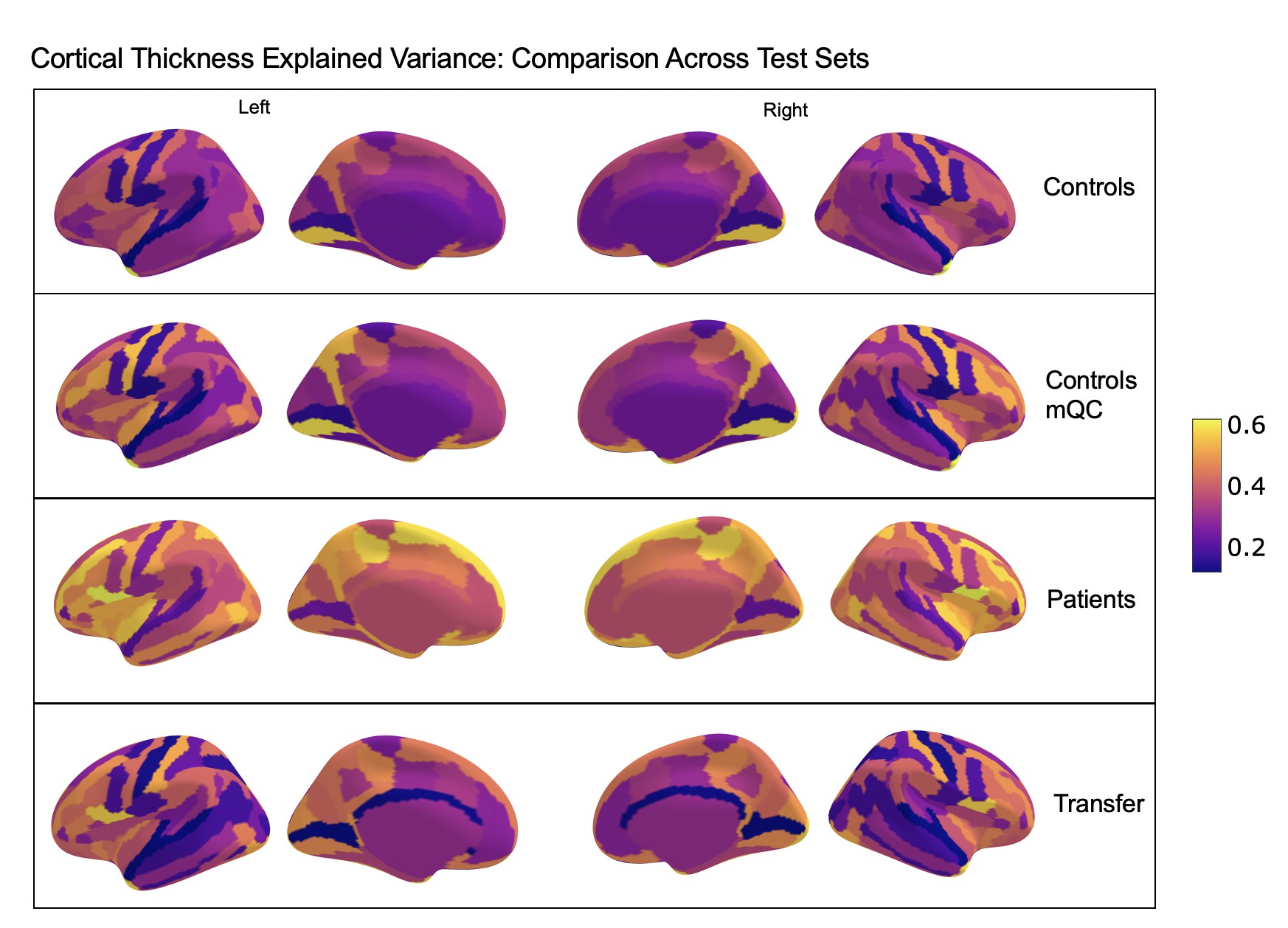
Comparison of the explained variance in cortical thickness across the different test sets.
The patterns appear to be robust and consistent across the different test sets.
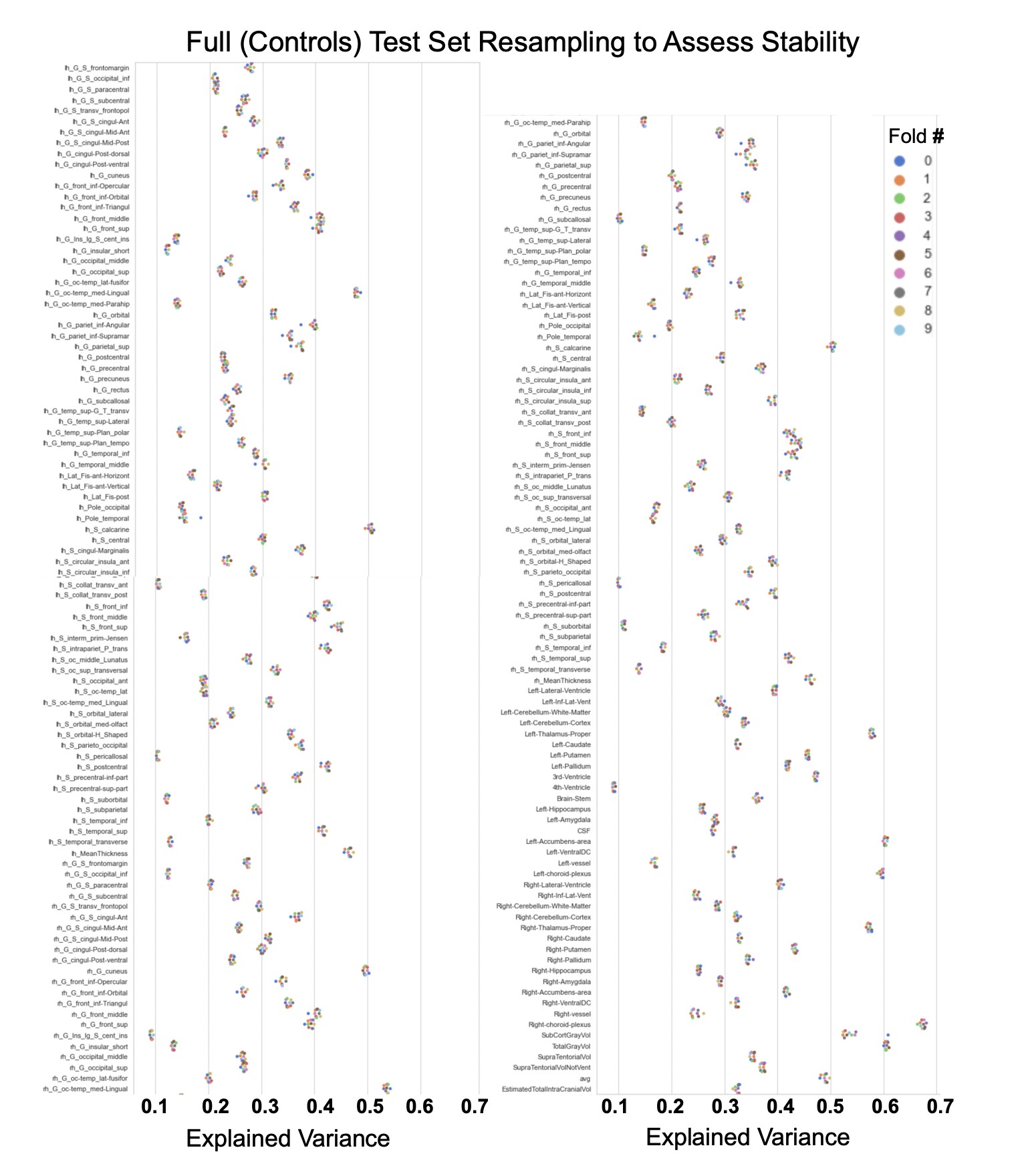
Showing the explained variance for each brain region across 10 randomized resampling of the full control test set.
Per site explained variance across the different test sets.
elife-72904-video1.mp4 video/mp4
We validate our models with several careful procedures: first, we report out of sample metrics; second, we perform a supplementary analysis on a subset of participants for whom input data had undergone manual quality checking by an expert rater (Table 1 – mQC). Third, each model fit was evaluated using metrics (Figure 3, Figure 3—figure supplements 1–3) that quantify central tendency and distributional accuracy 9Dinga et al.202114Fraza et al.2021. We also estimated separate models for males and females, which indicate that sex effects are adequately modeled using a global offset. Finally, to facilitate independent validation, we packaged pretrained models and code for transferring to new samples into an open resource for use by the community and demonstrated how to transfer the models to new samples (i.e., data not present in the initial training set).
Our models provide the opportunity for mapping the diverse trajectories of different brain areas. Several examples are shown in Figure 1C and D which align with known patterns of development and aging 10Ducharme et al.201616Gogtay et al.200440Tamnes et al.2010. Moreover, across the cortex and subcortex our model fits well, explaining up to 80% of the variance out of sample (Figure 3, Figure 3—figure supplements 1–3).
A goal of this work is to develop normative models that can be applied to many different clinical conditions. To showcase this, we apply the model to a transdiagnostic psychiatric cohort (Table 1 – Clinical; Figure 2) resulting in personalized, whole-brain deviation maps that can be used to understand inter-individual variability (e.g., for stratification) and to quantify group separation (e.g., case-control effects). To demonstrate this, for each clinical group, we summarized the individual deviations within that group by computing the proportion of subjects that have deviations in each region and comparing to matched (same sites) controls in the test set (Figure 2B–C). Additionally, we performed case-control comparisons on the raw cortical thickness and subcortical volumes, and on the deviation maps (Figure 2D), again against a matched sample from the test set. This demonstrates the advantages of using normative models for investigating individual differences in psychiatry, that is, quantifying clinically relevant information at the level of each individual. For most diagnostic groups, the z-statistics derived from the normative deviations also provided stronger case-control effects than the raw data. This shows the importance of accurate modeling of population variance across multiple clinically relevant dimensions. The individual-level deviations provide complimentary information to the group effects, which aligns with previous work 44Wolfers et al.201845Wolfers et al.202048Zabihi et al.2020. We note that a detailed description of the clinical significance of our findings is beyond the scope of this work and will be presented separately.
Discussion
In this work, we create lifespan brain charts of cortical thickness and subcortical volume derived from structural MRI, to serve as reference models. Multiple data sets were joined to build a mega-site lifespan reference cohort to provide good coverage of the lifespan. We applied the reference cohort models to clinical data sets and demonstrated the benefits of normative modeling in addition to standard case-control comparisons. All models, including documentation and code, are made available to the research community. We also provide an example data set (that includes data from sites not in the training sample) along with the code to demonstrate how well our models can adapt to new sites, and how easy it is to transfer our pretrained models to users’ own data sets.
We identify three main strengths of our approach. First, our large lifespan data set provides high anatomical specificity, necessary for discriminating between conditions, predicting outcomes, and stratifying subtypes. Second, our models are flexible in that they can model non-Gaussian distributions, can easily be transferred to new sites, and are built on validated analytical techniques and software tools 14Fraza et al.202123Kia et al.202128Marquand et al.2019. Third, we show the general utility of this work in that it provides the ability to map individual variation whilst also improving case-control inferences across multiple disorders.
In recent work, a large consortium established lifespan brain charts that are complementary to our approach 4Bethlehem et al.2021. Benefits of their work include precisely quantifying brain growth using a large cohort, but they only provide estimates of four coarse global measures (e.g., total brain volume). While this can precisely quantify brain growth and aging this does not provide the ability to generate individualized fingerprints or to stratify clinical cohorts. In contrast, in this work, we focus on providing spatially specific estimates (188 different brain regions) across the post-natal lifespan which provides fine-grained anatomical estimates of deviation, offering an individualized perspective that can be used for clinical stratification. We demonstrate the transdiagnostic clinical value of our models (Figure 2) by showing how clinical variation is widespread in a fine-grain manner (e.g., not all individuals deviate in the same regions and not all disorders have the same characteristic patterns) and we facilitate clinical applications of our models by sharing tutorial code notebooks with sample data that can be run locally or online in a web browser.
We also identify the limitations of this work. We view the word ‘normative’ as problematic. This language implies that there are normal and abnormal brains, a potentially problematic assumption. As indicated in Figure 2, there is considerable individual variability and heterogeneity among trajectories. We encourage the use of the phrase ‘reference cohort’ over ‘normative model’. In order to provide coverage of the lifespan the curated data set is based on aggregating existing data, meaning there is unavoidable sampling bias. Race, education, and socioeconomic variables were not fully available for all included data sets, however, given that data were compiled from research studies, they are likely samples drawn predominantly from Western, Educated, Industrialized, Rich, and Democratic (WEIRD) societies 18Henrich et al.2010 and future work should account for these factors. The sampling bias of UKBiobank 15Fry et al.2017 is especially important for users to consider as UKBiobank data contributes 59% of the full sample. By sampling both healthy population samples and case-control studies, we achieve a reasonable estimate of variation across individuals, however, downstream analyses should consider the nature of the reference cohort and whether it is appropriate for the target sample. Second, we have relied on semi-automated quality control (QC) for the full sample which—despite a conservative choice of inclusion threshold—does not guarantee either that low-quality data were excluded or that the data were excluded are definitively excluded because of artifacts. We addressed this by comparing our full test set to a manually quality check data set and observed similar model performance. Also, Freesurfer was not adjusted for the very young age ranges (2–7 yo) thus caution should be used when interpreting the model on new data in this age range. Finally, although the models presented in this study are comprehensive, they are only the first step, and we will augment our repository with more diverse data, different features, and modeling advances as these become available.
Materials and methods
Data from 82 sites were combined to create the initial full sample. These sites are described in detail in Supplementary files 1-2, including the sample size, age (mean and standard deviation), and sex distribution of each site. Many sites were pulled from publicly available data sets including ABCD, ABIDE, ADHD200, CAMCAN, CMI-HBN, HCP-Aging, HCP-Development, HCP-Early Psychosis, HCP-Young Adult, IXI, NKI-RS, Oasis, OpenNeuro, PNC, SRPBS, and UKBiobank. For data sets that include repeated visits (i.e., ABCD and UKBiobank), only the first visit was included. Other included data come from studies conducted at the University of Michigan 11Duval et al.201835Rutherford et al.202041Tomlinson et al.202042Tso et al.202143Weigard et al.202149Zucker et al.2009, University of California Davis 32Nordahl et al.2020, University of Oslo 31Nesvåg et al.2017, King’s College London 17Green et al.201225Lythe et al.2015, and Amsterdam University Medical Center 29Mocking et al.2016. Full details regarding sample characteristics, diagnostic procedures, and acquisition protocols can be found in the publications associated with each of the studies. Equal sized training and testing data sets (split half) were created using scikit-learn’s train_test_split function, stratifying on the site variable. It is important to stratify based on site, not only study 4Bethlehem et al.2021, as many of the public studies (i.e., ABCD) include several sites, thus modeling study does not adequately address MRI scanner confounds. To test stability of the model performance, the full test set was randomly resampled 10 times and evaluation metrics were re-calculated on each split of the full test set (Figure 3—figure supplement 2). To show generalizability of the models to new data not included in training, we leveraged data from OpenNeuro.org 26Markiewicz et al.2021 to create a transfer data set (six sites, N=546, Supplementary file 3). This data are provided along with the code for transferring to walk users through how to apply these models to their own data.
The clinical validation sample consisted of a subset of the full data set (described in detail in Figure 1A, Figure 2A and Supplementary file 1). Studies (sites) contributing clinical data included: Autism Brain Imaging Database Exchange (ABIDE GU, KKI, NYU, USM), ADHD200 (KKI, NYU), CNP, SRPBS (CIN, COI, KTT, KUT, HKH, HRC, HUH, SWA, UTO), Delta (AmsterdamUMC), Human Connectome Project Early Psychosis (HCP-EP BWH, IU, McL, MGH), KCL, University of Michigan Children Who Stutter (UMich_CWS), University of Michigan Social Anxiety Disorder (UMich_SAD), University of Michigan Schizophrenia Gaze Processing (UMich_SZG), and TOP (University of Oslo).
In addition to the sample-specific inclusion criteria, inclusion criteria for the full sample were based on participants having basic demographic information (age and sex), a T1-weighted MRI volume, and Freesurfer output directories that include summary files that represent left and right hemisphere cortical thickness values of the Destrieux parcellation and subcortical volumetric values (aseg.stats, lh.aparc.a2009s.stats, and rh.aparc.a2009s.stats). Freesurfer image analysis suite (version 6.0) was used for cortical reconstruction and volumetric segmentation for all studies. The technical details of these procedures are described in prior publications 6Dale et al.199913Fischl et al.200212Fischl and Dale2000. UK Biobank was the only study for which Freesurfer was not run by the authors. Freesurfer functions aparcstats2table and asegstats2table were run to extract cortical thickness from the Destrieux parcellation 7Destrieux et al.2010 and subcortical volume for all participants into CSV files. These files were inner merged with the demographic files, using Pandas, and NaN rows were dropped.
QC is an important consideration for large samples and is an active research area 1Alfaro-Almagro et al.201824Klapwijk et al.201934Rosen et al.2018. We consider manual quality checking of images both prior to and after preprocessing to be the gold standard. However, this is labor intensive and prohibitive for very large samples. Therefore, in this work, we adopt a pragmatic and multi-pronged approach to QC. First, a subset of the full data set underwent manual quality checking (mQC) by author S.R. Papaya, a JavaScript-based image viewer. Manual quality checking was performed during December 2020 when the Netherlands was in full lockdown due to COVID-19 and S.R. was living alone in a new country with a lot of free time. Data included in this manual QC step was based on what was available at the time (Supplementary file 2). Later data sets that were included were not manually QC’d due to resource and time constraints. Scripts were used to initialize a manual QC session and track progress and organize ratings. All images (T1w volume and Freesurfer brain.finalsurfs) were put into JSON files that the mQC script would call when loading Papaya. Images were rated using a ‘pass/fail/flag’ scale and the rating was tracked in an automated manner using keyboard inputs (up arrow=pass, down arrow=fail, F key=flag, and left/right arrows were used to move through subjects). Each subject’s T1w volume was viewed in 3D volumetric space, with the Freesurfer brain.finalsurfs file as an overlay, to check for obvious quality issues such as excessive motion, ghosting or ringing artifacts. Example scripts used for quality checking and further instructions for using the manual QC environment can be found on GitHub(38Rutherford2022 copy archived at swh:1:rev:70894691c74febe2a4d40ab0c84c50094b9e99ce). We relied on ABCD consortium QC procedures for the QC for this sample. The ABCD study data distributes a variable (freesqc01.txt; fsqc_qc = = 1/0) that represents manual quality checking (pass/fail) of the T1w volume and Freesurfer data, thus this data set was added into our manual quality checked data set bringing the sample size to 24,354 individuals passing manual quality checks. Note that QC was performed on the data prior to splitting of the data to assess generalizability. Although this has a reduced sample, we consider this to be a gold-standard sample in that every single scan has been checked manually. All inferences reported in this manuscript were validated against this sample. Second, for the full sample, we adopted an automated QC procedure that quantifies image quality based on the Freesurfer Euler Characteristic (EC), which has been shown to be an excellent proxy for manual labeling of scan quality 30Monereo-Sánchez et al.202134Rosen et al.2018 and is the most important feature in automated scan quality classifiers 24Klapwijk et al.2019. Since the distribution of the EC varies across sites, we adopt a simple approach that involves scaling and centering the distribution over the EC across sites and removing samples in the tail of the distribution (see 23Kia et al.2021 for details). While any automated QC heuristic is by definition imperfect, we note that this is based on a conservative inclusion threshold such that only samples well into the tail of the EC distribution are excluded, which are likely to be caused by true topological defects rather than abnormalities due to any underlying pathology. We separated the evaluation metrics into full test set (relying on automated QC) and mQC test set in order to compare model performance between the two QC approaches and were pleased to notice that the evaluation metrics were nearly identical across the two methods.
Normative modeling was run using python 3.8 and the PCNtoolkit package (version 0.20). Bayesian Linear Regression (BLR) with likelihood warping was used to predict cortical thickness and subcortical volume from a vector of covariates (age, sex, and site). For a complete mathematical description and explanation of this implementation, see 14Fraza et al.2021. Briefly, for each brain region of interest (cortical thickness or subcortical volume), is predicted as:
where is the estimated weight vector, is a basis expansion of the of covariate vector x, consisting of a B-spline basis expansion (cubic spline with five evenly spaced knots) to model non-linear effects of age, and a Gaussian noise distribution with mean zero and noise precision term β (the inverse variance). A likelihood warping approach 33Rios and Tobar201939Snelson et al.2003 was used to model non-Gaussian effects. This involves applying a bijective non-linear warping function to the non-Gaussian response variables to map them to a Gaussian latent space where inference can be performed in closed form. We employed a ‘sinarcsinsh’ warping function, which is equivalent to the SHASH distribution commonly used in the generalized additive modeling literature 21Jones and Pewsey2009 and which we have found to perform well in prior work 9Dinga et al.202114Fraza et al.2021. Site variation was modeled using fixed effects, which we have shown in prior work provides relatively good performance 23Kia et al.2021, although random effects for site may provide additional flexibility at higher computational cost. A fast numerical optimization algorithm was used to optimize hyperparameters (L-BFGS). Computational complexity of hyperparameter optimization was controlled by minimizing the negative log-likelihood. Deviation scores (Z-scores) are calculated for the n-th subject, and d-th brain area, in the test set as:
Where is the true response, is the predicted mean, is the estimated noise variance (reflecting uncertainty in the data), and is the variance attributed to modeling uncertainty. Model fit for each brain region was evaluated by calculating the explained variance (which measures central tendency), the mean squared log-loss (MSLL, central tendency, and variance) plus skew and kurtosis of the deviation scores (2) which measures how well the shape of the regression function matches the data 9Dinga et al.2021. Note that for all models, we report out of sample metrics.
To provide a summary of individual variation within each clinical group, deviation scores were summarized for each clinical group (Figure 2B–C) by first separating them into positive and negative deviations, counting how many subjects had an extreme deviation (positive extreme deviation defined as Z>2, negative extreme deviation as Z<−2) at a given ROI, and then dividing by the group size to show the percentage of individuals with extreme deviations at that brain area. Controls from the same sites as the patient groups were summarized in the same manner for comparison. We also performed classical case versus control group difference testing on the true data and on the deviation scores (Figure 2D) and thresholded results at a Benjamini-Hochberg false discovery rate of p<0.05. Note that in both cases, we directly contrast each patient group to their matched controls to avoid nuisance variation confounding any reported effects (e.g., sampling characteristics and demographic differences).
All pretrained models and code are shared online with straightforward directions for transferring to new sites and including an example transfer data set derived from several OpenNeuro.org data sets. Given a new set of data (e.g., sites not present in the training set), this is done by first applying the warp parameters estimating on the training data to the new data set, adjusting the mean and variance in the latent Gaussian space, then (if necessary) warping the adjusted data back to the original space, which is similar to the approach outlined in 9Dinga et al.2021. Note that to remain unbiased, this should be done on a held-out calibration data set. To illustrate this procedure, we apply this approach to predicting a subset of data that was not used during the model estimation step. We leveraged data from OpenNeuro.org 26Markiewicz et al.2021 to create a transfer data set (six sites, N=546, Supplementary file 3). This data are provided along with the code for transferring to walk users through how to apply these models to their own data. These results are reported in Figure 3 (transfer) and Supplementary file 3. We also distribute scripts for this purpose in the GitHub Repository associated with this manuscript. Furthermore, to promote the use of these models and remove barriers to using them, we have set up access to the pretrained models and code for transferring to users’ own data, using Google Colab, a free, cloud-based platform for running python notebooks. This eliminates the need to install python/manage package versions and only requires users to have a personal computer with stable internet connection.
References
-
- FAlfaro-Almagro
- MJenkinson
- NKBangerter
- JLRAndersson
- LGriffanti
- GDouaud
- SNSotiropoulos
- SJbabdi
- MHernandez-Fernandez
- EVallee
- DVidaurre
- MWebster
- PMcCarthy
- CRorden
- ADaducci
- DCAlexander
- HZhang
- IDragonu
- PMMatthews
- KLMiller
- SMSmith
- doi10.1016/j.neuroimage.2017.10.034
- pmid29079522
-
- JMMBayer
- RDinga
- SMKia
- ARKottaram
- TWolfers
- JLv
- AZalesky
- LSchmaal
- AMarquand
- doi10.1101/2021.02.09.430363
-
- RAIBethlehem
- JSeidlitz
- RRomero-Garcia
- STrakoshis
- GDumas
- MVLombardo
- doi10.1038/s42003-020-01212-9
- pmid32887930
-
- RBethlehem
- JSeidlitz
- SRWhite
- JWVogel
- KMAnderson
- CAdamson
- SAdler
- GSAlexopoulos
- EAnagnostou
- AAreces-Gonzalez
- DEAstle
- BAuyeung
- MAyub
- GBall
- SBaron-Cohen
- RBeare
- SABedford
- VBenegal
- FBeyer
- AFAlexander-Bloch
- doi10.1101/2021.06.08.447489
-
- TJCole
- doi10.3109/03014460.2012.694475
- pmid22780429
-
- AMDale
- BFischl
- MISereno
- doi10.1006/nimg.1998.0395
- pmid9931268
-
- CDestrieux
- BFischl
- ADale
- EHalgren
- doi10.1016/j.neuroimage.2010.06.010
- pmid20547229
-
- RDimitrova
- MPietsch
- DChristiaens
- JCiarrusta
- TWolfers
- DBatalle
- EHughes
- JHutter
- LCordero-Grande
- ANPrice
- AChew
- SFalconer
- KVecchiato
- JKSteinweg
- OCarney
- MARutherford
- J-DTournier
- SJCounsell
- AFMarquand
- DRueckert
- JVHajnal
- GMcAlonan
- ADEdwards
- JO’Muircheartaigh
- doi10.1093/cercor/bhaa069
- pmid32306044
-
- RDinga
- CJFraza
- JMMBayer
- SMKia
- CFBeckmann
- AFMarquand
- doi10.1101/2021.06.14.448106
-
- SDucharme
- MDAlbaugh
- TVNguyen
- JJHudziak
- JMMateos-Pérez
- ALabbe
- ACEvans
- SKarama
- Brain Development Cooperative Group
- doi10.1016/j.neuroimage.2015.10.010
- pmid26463175
-
- ERDuval
- SAJoshi
- SRussmanBlock
- JLAbelson
- ILiberzon
- doi10.1016/j.janxdis.2018.04.004
- pmid29729828
-
- BFischl
- AMDale
- doi10.1073/pnas.200033797
- pmid10984517
-
- BFischl
- DHSalat
- EBusa
- MAlbert
- MDieterich
- CHaselgrove
- AvanderKouwe
- RKilliany
- DKennedy
- SKlaveness
- AMontillo
- NMakris
- BRosen
- AMDale
- doi10.1016/S0896-6273(02)00569-X
- pmid11832223
-
- CJFraza
- RDinga
- CFBeckmann
- AFMarquand
- doi10.1016/j.neuroimage.2021.118715
- pmid34798518
-
- AFry
- TJLittlejohns
- CSudlow
- NDoherty
- LAdamska
- TSprosen
- RCollins
- NEAllen
- doi10.1093/aje/kwx246
- pmid28641372
-
- NGogtay
- JNGiedd
- LLusk
- KMHayashi
- DGreenstein
- ACVaituzis
- TFNugent
- DHHerman
- LSClasen
- AWToga
- JLRapoport
- PMThompson
- doi10.1073/pnas.0402680101
- pmid15148381
-
- SGreen
- MALambonRalph
- JMoll
- JFWDeakin
- RZahn
- doi10.1001/archgenpsychiatry.2012.135
- pmid22638494
-
- JHenrich
- SJHeine
- ANorenzayan
- doi10.1017/S0140525X0999152X
- pmid20550733
-
- TRInsel
- doi10.1001/jama.2014.1193
- pmid24794359
-
- CRJack
- DSKnopman
- WJJagust
- LMShaw
- PSAisen
- MWWeiner
- RCPetersen
- JQTrojanowski
- doi10.1016/S1474-4422(09)70299-6
- pmid20083042
-
- MCJones
- APewsey
- doi10.1093/biomet/asp053
-
- GBKaras
- PScheltens
- SARBRombouts
- PJVisser
- RAvanSchijndel
- NCFox
- FBarkhof
- doi10.1016/j.neuroimage.2004.07.006
- pmid15488420
-
- SMKia
- HHuijsdens
- SRutherford
- RDinga
- TWolfers
- MMennes
- OAAndreassen
- LTWestlye
- CFBeckmann
- AFMarquand
- doi10.1101/2021.05.28.446120
-
- ETKlapwijk
- FvandeKamp
- MvanderMeulen
- SPeters
- LMWierenga
- doi10.1016/j.neuroimage.2019.01.014
- pmid30633965
-
- KELythe
- JMoll
- JAGethin
- CIWorkman
- SGreen
- MALambonRalph
- JFWDeakin
- RZahn
- doi10.1001/jamapsychiatry.2015.1813
- pmid26445229
-
- CJMarkiewicz
- KJGorgolewski
- FFeingold
- RBlair
- YOHalchenko
- EMiller
- NHardcastle
- JWexler
- OEsteban
- MGoncavles
- AJwa
- RPoldrack
- doi10.7554/eLife.71774
- pmid34658334
-
- AFMarquand
- IRezek
- JBuitelaar
- CFBeckmann
- doi10.1016/j.biopsych.2015.12.023
- pmid26927419
-
- AFMarquand
- SMKia
- MZabihi
- TWolfers
- JKBuitelaar
- CFBeckmann
- doi10.1038/s41380-019-0441-1
- pmid31201374
-
- RJTMocking
- CAFigueroa
- MMRive
- HGeugies
- MNServaas
- JAssies
- MWJKoeter
- FMVaz
- MWichers
- JPvanStraalen
- RdeRaedt
- CLHBockting
- CJHarmer
- AHSchene
- HGRuhé
- doi10.1136/bmjopen-2015-009510
- pmid26932139
-
- JMonereo-Sánchez
- JJAdeJong
- GSDrenthen
- MBeran
- WHBackes
- CDAStehouwer
- MTSchram
- DEJLinden
- JFAJansen
- doi10.1016/j.neuroimage.2021.118174
- pmid34000406
-
- RNesvåg
- EGJönsson
- IJBakken
- GPKnudsen
- TDBjella
- TReichborn-Kjennerud
- IMelle
- OAAndreassen
- doi10.1186/s12888-017-1256-8
- pmid28292279
-
- CWNordahl
- AMIosif
- GSYoung
- AHechtman
- BHeath
- JKLee
- LLibero
- VPReinhardt
- BWinder-Patel
- DGAmaral
- SRogers
- MSolomon
- SOzonoff
- doi10.1016/j.jaac.2019.11.022
- pmid31972262
-
- GRios
- FTobar
- doi10.1016/j.neunet.2019.06.012
- pmid31319321
-
- AFGRosen
- DRRoalf
- KRuparel
- JBlake
- KSeelaus
- LPVilla
- RCiric
- PACook
- CDavatzikos
- MAElliott
- AGarciadeLaGarza
- EDGennatas
- MQuarmley
- JESchmitt
- RTShinohara
- MDTisdall
- RCCraddock
- REGur
- RCGur
- TDSatterthwaite
- doi10.1016/j.neuroimage.2017.12.059
- pmid29278774
-
- SRutherford
- MAngstadt
- CSripada
- SEChang
- doi10.1101/2020.10.28.359711
-
- SRutherford
-
- SRutherford
-
- SRutherford
-
- ESnelson
- CERasmussen
- ZGhahramani
-
- CKTamnes
- YOstby
- AMFjell
- LTWestlye
- PDue-Tønnessen
- KBWalhovd
- doi10.1093/cercor/bhp118
- pmid19520764
-
- RCTomlinson
- SABurt
- RWaller
- JJonides
- ALMiller
- ANGearhardt
- SJPeltier
- KLKlump
- JCLumeng
- LWHyde
- doi10.1016/j.neuroimage.2020.116536
- pmid31935521
-
- IFTso
- MAngstadt
- SRutherford
- SPeltier
- VADiwadkar
- SFTaylor
- doi10.1016/j.schres.2020.11.012
- pmid33229223
-
- ASWeigard
- SJBrislin
- LMCope
- JEHardee
- MEMartz
- ALy
- RAZucker
- CSripada
- MMHeitzeg
- doi10.1007/s00213-021-05885-w
- pmid34173032
-
- TWolfers
- NTDoan
- TKaufmann
- DAlnæs
- TMoberget
- IAgartz
- JKBuitelaar
- TUeland
- IMelle
- BFranke
- OAAndreassen
- CFBeckmann
- LTWestlye
- AFMarquand
- doi10.1001/jamapsychiatry.2018.2467
- pmid30304337
-
- TWolfers
- CFBeckmann
- MHoogman
- JKBuitelaar
- BFranke
- AFMarquand
- doi10.1017/S0033291719000084
- pmid30782224
-
- TWolfers
- JRokicki
- DAlnaes
- PBerthet
- IAgartz
- SMKia
- TKaufmann
- MZabihi
- TMoberget
- IMelle
- CFBeckmann
- OAAndreassen
- AFMarquand
- LTWestlye
- doi10.1002/hbm.25386
- pmid33638594
-
- M.Zabihi
- MOldehinkel
- TWolfers
- VFrouin
- DGoyard
- ELoth
- TCharman
- JTillmann
- TBanaschewski
- GDumas
- RHolt
- SBaron-Cohen
- SDurston
- SBölte
- DMurphy
- CEcker
- JKBuitelaar
- CFBeckmann
- AFMarquand
- doi10.1016/j.bpsc.2018.11.013
- pmid30799285
-
- MZabihi
- DLFloris
- SMKia
- TWolfers
- JTillmann
- ALArenas
- CMoessnang
- TBanaschewski
- RHolt
- SBaron-Cohen
- ELoth
- TCharman
- TBourgeron
- DMurphy
- CEcker
- JKBuitelaar
- CFBeckmann
- AMarquand
- EU-AIMS LEAP Group
- doi10.1038/s41398-020-01057-0
- pmid33159037
-
- RAZucker
- DAEllis
- HEFitzgerald
- CRBingham
- KSanford
- doi10.1017/S0954579400007458